Correction, Corrective Action, and Preventive Action (CCAPA) are essential management systems components that help organizations identify, address, and prevent issues to ensure ongoing process improvement.
Table of Contents:
Click to expand
Confusion When Addressing Nonconformances or Noncompliances
Some individuals or organizations, unaware of the difference between the three terms, sometimes think that corrective Action is the only option. Others have Management System documents that frequently mention CAPAs even though Preventive Action is no longer a formal part of their system.
Corrective Action is not always needed; sometimes, a Correction alone is enough to address a Nonconformity.
Definition of Terms
To understand CCAPA better, it is essential to understand several key terms and concepts within quality management and organizational improvement. Here's a list of terms, taken from the ISO Standards, you may want to familiarize yourself with:
Correction: ISO 9001:2015 defines Correction as the Action to eliminate a detected nonconformity. It can be "made in advance of, in conjunction with, or after corrective action."
Corrective Action: ISO 9000:2015 defines Corrective Action as eliminating the cause of nonconformity and preventing its recurrence.
Preventive Action: ISO 9000:2015 defines Preventive Action as Action taken to eliminate the cause of a potential nonconformity or other potential undesirable situation
Incidents: An incident refers to an unexpected or unplanned event that deviates from normal operations, processes, or expectations within an organization
Nonconformity: Any deviation, defect, or discrepancy observed in a product, process, or system that does not meet specified requirements, standards, or expectations.
Non-compliance: Refers to the failure to adhere to laws, regulations, rules, policies, or standards set by external governing bodies, regulatory authorities, or industry-specific requirements.
Root Cause Analysis: A systematic process used to identify the fundamental underlying cause(s) of a problem or nonconformity rather than just addressing its symptoms.
Continuous Improvement: A philosophy and methodology focused on enhancing products, processes, and systems through incremental changes and innovations.
Process Improvement: The systematic approach of enhancing processes to improve efficiency, effectiveness, and quality.
Risk Assessment: Identifying, analyzing, and evaluating potential risks impacting the organization's objectives or operations.
Compliance: Adherence to laws, regulations, standards, and internal policies relevant to an organization's operations and industry.
What ISO Standards Say About CCAPA
ISO's requirements on CCAPA provide a structured guide for effectively managing nonconformities, process improvements, and regulatory compliance. Here's a quick overview of what some frequently used ISO standards have to say.
ISO 13485
Let's start with the 'outlier.' ISO 13485 follows the structure of the 2008 version of the ISO 9001 standard, not the current 2015 version. Some significant differences relating to CCAPA arise.
It is difficult to find mention of the Correction of ISO Management Systems (MS) Standards. It was mentioned in ISO 13485 Clause 8.2.2: Complaint Handling, where we find Corrections in "f) determining the need to initiate corrections or corrective actions" and in Clause 8.5.2, where the need for documented procedures for implementing corrective actions was emphasized.
Preventive Action, for its part, was omitted from the first HLS (High-Level Structure) document issued in 2010 as a guide to the ISO Committees drafting new and revised management system standards; however, ISO 13485:2016 dHow to Check the Effectiveness of Corrective Actionid not adopt the HLS Structure and retained the previous ISO 9001:2008 structure instead.
To illustrate, Clause 8.5.3 still focuses on preventive actions to address potential issues (while the 2016 version of ISO 13485 removed the specific requirement for a separate documented procedure for preventive Action, organizations are still encouraged to undertake proactive measures to prevent problems from occurring).
ISO 9001
Many persons working in the field claim that Preventive Action has been removed from ISO Standards, but it's actually there if you look hard enough.
Take ISO 9001:2015 Clause 10.2.1 b) 3) - yes, you have to drill down a bit! – wherein evaluating the need for Action to eliminate the cause(s) of the nonconformity (so that it does not recur or occur elsewhere) requires…
'determining if similar nonconformities exist or could potentially occur.'
The phrase 'or could potentially occur' is directly equivalent to preventive Action.
Some organizations that have been certified for many years are comfortable with Preventive Action and retain it as part of their Management System even though it is not included in the MS Standard to which they are certified (This is not a problem as nothing in any HSL-based Standards says you can't retain it. Make sure your Management System Documentation clearly distinguishes Corrections from Corrective Action).
ISO 14001
ISO 14001 Clause 10.2 requires identifying nonconformities and asking for the implementation of corrective actions to correct deviations from environmental policies or objectives.
Moreover, ISO 14001 stresses the importance of preventive actions to minimize severe environmental impacts and prevent future nonconformity.
ISO 27001
ISO 27001 Clause 10.1 focuses on managing information security incidents by setting up processes to handle nonconformities and initiate corrective actions. It emphasizes explicitly analyzing nonconformities, identifying root causes, and implementing corrective measures to enhance information security.
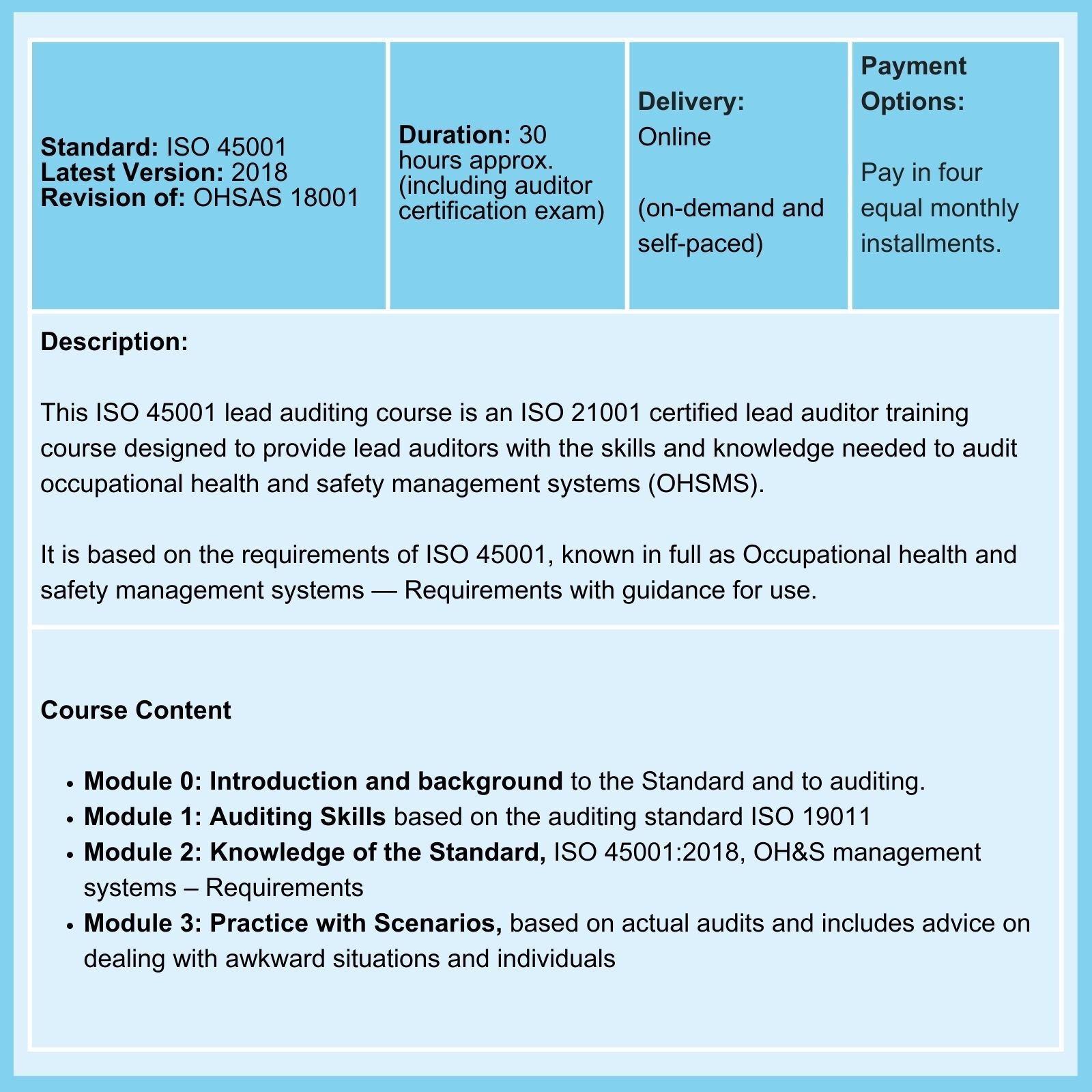
ISO 45001
Lastly, ISO 45001 Clause 10.2 underlines the importance of addressing incidents and nonconformities while stressing the need for corrective and preventive actions to enhance occupational health and safety. It requires thorough investigations into incidents, identifying root causes, implementing corrective measures, and proactive planning for preventative actions to mitigate risks effectively.
Examples of Correction, Corrective Action, and Preventive Action
To better understand how Correction, Corrective Action, and Preventive Action compare, here's a table showing sample scenarios from different industries and what actions qualify as Correction, Corrective Action, or Preventive Action.
Correction |
Corrective Action |
Prevention |
|---|---|---|
|
Halting production to fix a machine malfunction causing product defects. The assembly line stops immediately as technicians work to repair the faulty machine to ensure no more defective items are produced. |
Conducting a root cause analysis and redesigning a faulty production process to prevent recurring defects. Engineers analyze the production line, identify the flaws, and implement new procedures to prevent similar defects from arising in the future. |
Implementing predictive maintenance schedules to prevent equipment breakdowns. The manufacturing plant schedules regular maintenance checks based on equipment performance data to avoid unexpected machine failures. |
|
Providing immediate medical care to a patient experiencing adverse reactions to medication. Nurses and doctors swiftly administer the necessary treatment to alleviate the patient's symptoms and prevent further health complications. |
Implementing additional staff training after an analysis reveals consistent errors in patient record-keeping. The healthcare facility conducts specialized training sessions to ensure accurate and compliant patient documentation. |
Offering preventative health screenings to identify potential health issues early. Healthcare providers conduct routine screenings and tests to detect health problems in their early stages, enabling timely interventions. |
|
Restarting a server to resolve a sudden system outage or temporary disruption. IT specialists quickly identify the server issue and reboot it, restoring normal operations and minimizing user downtime. |
Installing security patches and updating protocols to prevent recurrent cyber-attacks. IT teams review the system vulnerabilities, install necessary patches, and enforce updated security measures to bolster the network against future attacks. |
Regularly backing up data and implementing robust cybersecurity measures to prevent data loss or breaches. The IT department regularly backs up critical data and deploys multifaceted security measures to safeguard against data breaches. |
|
Repairing a structural defect found during building inspections before continuing construction. Construction workers immediately stop their work to fix the identified structural flaw to ensure the building's integrity and safety. |
Revamping safety protocols and providing additional safety equipment after an accident investigation. The construction company overhauls safety guidelines and equips workers with advanced protective gear to prevent similar accidents. |
Providing comprehensive safety training programs for all workers to prevent accidents. The construction firm conducts ongoing safety training sessions to educate workers on potential hazards and safe work practices. |
|
Quickly refunding a customer for a wrong item delivered to address the immediate dissatisfaction. Customer service representatives promptly issue a refund to resolve the customer's complaint and maintain a positive relationship. |
Enhancing quality control checks in the warehouse to prevent future shipping errors. The retail company implements more stringent quality inspections before shipping products to ensure accurate orders. |
Collaborating with suppliers to conduct quality checks before receiving products to prevent selling defective items. Retailers work closely with suppliers to ensure high-quality products are delivered by performing rigorous quality checks before accepting shipments. |
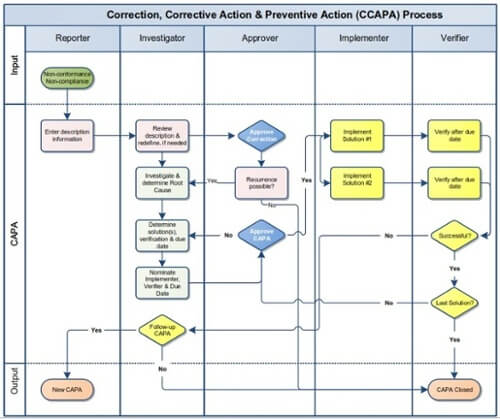
The CCAPA Process for Dealing with Nonconformances or Noncompliances
The CCAPA process is like a cycle that helps fix problems and prevent them from happening again. It deals with issues by figuring out why they happened, fixing them, and taking Action to ensure they don't happen again. Below is a graphic of the overall CCAPA Process for treating a nonconformance and brief explanations of the steps involved.
Click the image for a copy of the infographic.
Correction Process
- Identification of Nonconformance/Non-Compliance - Recognize and document the specific instance or instances of nonconformance or non-compliance, whether it's a product defect, process deviation, regulatory violation, or failure to meet standards.
- Immediate Action (Correction) - Implement quick actions (corrections) to address the immediate symptoms or issues to prevent further escalation or immediate harm. Corrections are temporary measures taken to contain the problem until a more thorough investigation can be conducted.
Corrective Action Process
- Root Cause Analysis - Investigate the identified nonconformance/non-compliance to determine the issue's root cause(s). This involves a thorough analysis to understand why the problem occurred.
- Development and Implementation of Corrective Actions - Based on the root cause analysis, develop and implement corrective actions to address the underlying cause(s) of the nonconformance/non-compliance. These actions are focused on eliminating the root cause to prevent recurrence.
- Documentation and Monitoring - Document the corrective actions taken and monitor their implementation to ensure effectiveness. Tracking progress and verifying that the corrective measures resolve the issue is essential.
Preventive Action Process
- Risk Assessment and Prevention Planning—Conduct a risk assessment to identify potential future risks or similar nonconformances/non-compliances. Develop preventive action plans to prevent similar issues from occurring in the future.
- Implementation of Preventive Actions - Implement proactive measures (preventive actions) designed to mitigate identified risks and prevent the recurrence of similar nonconformances/non-compliances. These actions focus on improving processes or systems to prevent future occurrences.
- Monitoring and Review - Continuously monitor and review the effectiveness of preventive actions implemented. Regular reviews ensure that the preventive measures are successful in preventing similar issues.
Continuous Improvement
- Evaluation and Review - Assess the overall effectiveness of the CCAPA process. Evaluate whether the implemented corrective and preventive actions have successfully addressed the root cause and prevented recurrence.
- Documentation and Feedback Loop - Document all steps taken throughout the CCAPA process. Use feedback from the process to improve the organization's processes, systems, or procedures.
CCAPA Tools
CCAPA tools refer to the methodologies, software apps, and frameworks used in Correction, Corrective Action, and Preventive Action processes in quality management systems. These tools are crucial in identifying, addressing, and preventing nonconformities and improving organizational performance. Some common CCAPA tools and their importance include:
Corrective Action Reports (CARs) and Preventive Action Reports (PARs) - These reports document identified issues and root causes, implement corrective actions, and take measures to prevent recurrence. They provide a structured approach to problem-solving and continual improvement.
CAPA Software/Systems - Specialized software or systems that manage Corrective and Preventive Action processes. They streamline workflow, facilitate collaboration, track actions, and ensure compliance with regulatory requirements.
Root Cause Analysis (RCA) Tools - Various methodologies (e.g., Fishbone diagrams, 5 Whys, Fault Tree Analysis) identify the root causes of problems or nonconformities. They help in understanding the deeper reasons behind issues for effective corrective actions.
Quality Management Software (QMS) - Comprehensive software solutions encompass various quality management functions, including CAPA management, document control, risk management, audits, and compliance tracking.
Six Sigma and Lean Tools—Tools like DMAIC (Define, Measure, Analyze, Improve, Control) and PDCA (Plan, Do, Check, Act) help solve problems, improve processes, and prevent future issues by enhancing operational efficiency.
8-D (Eight Disciplines) - While not exclusively a CCAPA tool, the 8-D methodology is considered a problem-solving tool that integrates preventive measures to stop similar problems from happening again. Its structured, eight-step approach to identifying, correcting, and preventing problems is closely related to CCAPA principles.
And finally…
The effective application of Correction, Corrective Action, and Preventive Action is necessary to achieve organizational excellence; however, this will only be possible if organizations fully understand the full range of options and tools.
Mistakes happen in every organization, but with effective CCAPA processes, you can prevent recurrence, avoid unnecessary work, protect your reputation, improve customer satisfaction, and increase profit.
deGRANDSON Global is an ISO Certified Educational Organization
In October 2021, we secured certification to three education-related ISO Standards. We now have a university-grade management system in place conforming to the requirements of …
We have chosen ISO 21001 certification because, unlike IRCA and Exemplar badges (which, in our opinion, are commercially compromised), it is based on independent third-party assessment. It is a global standard that schools, colleges, and universities use to demonstrate competence.
We provide Courses for ISO 9001, ISO 13485, ISO 14001, ISO 17025, ISO 27001, ISO 45001, Risk Management, Data Protection, and many more.