ISO 9001 and other Management System Standards, 2025 to 2030
While there are more than 50 ISO Management System Standards, only about twelve are frequently used. It is these few that we will concentrate on in this post. While many new revisions of these standards are planned, the nature and significance of those changes have yet to emerge. Much will depend on the new revision of ISO 9001, which has an expected publication date of September 2026, as many other standards (e.g., ISO 14001) are based on ISO 9001 or are sectoral variants also based on ISO 9001 (e.g., IATF 16949).
The table below sets out the position as we understand it today. The information will be updated as firmer information becomes available, and the nature of proposed changes is clarified.
We invite you to tag this page and return from time to time for the latest news.
Don't believe the Transition Fairytales.
Every time a standard is revised, the rumor mill starts to talk of the possibility of 1-year, 2-year, or 3-year transition periods. The chosen periods are decided by either the IAF or by the sectoral organizations concerned. Two simple rules have evolved:
Rule 1: For all ISO Standards (e.g., ISO 45001), the transition period will be three years from the date of publication, and
Rule 2: For all Sectoral Standards (e.g., IS9100), the transition period will be two years from the date of publication. Exceptionally, a 1-year transition has been used, but 2 years is the norm.
The Stages of development of new/revised Standards
This is the sequence followed by ISO Technical Committees in developing Standards:
- WG – A Working Group is established;
- CD – A Committee Draft is published with initial thoughts on changes;
- DIS – A Draft International Standard is prepared, firming up on proposed changes;
- DIS2 – If the DIS has proved contentious, a second draft standard may be needed;
- FDIS – This Final Draft International Standard is the version it is proposed to publish; a revision to an FDIS has never occurred;
- ISO – The new Standard is published.
State of play as of 07-Nov-25
NOTE: The New Version dates are based on the best information currently available and are subject to change.
|
Standard |
Current Version |
New Version |
Comment |
|
ISO 9001 |
2015 |
Sep 2026 |
This Quality Management System Standard (better thought of as a Business Management Standard) may require a second DIS - ISO Committee Members are reportedly dissatisfied with the proposed changes. However, there is sufficient time to meet the target publication date. FDIS is scheduled for publication in March 2026. |
|
ISO 14001 |
2015 |
Apr 2026 |
The FDIS version was published in January 2026 in advance of ISO 9001:2026. |
|
ISO 27001 |
2022 |
None |
The transition period for the 2022 version ends on October 31, 2025. |
|
ISO 42001 |
2023 |
None |
This AI Management System Standard is generally considered technically weak, and an early revision is anticipated. It could be as soon as 2027. |
|
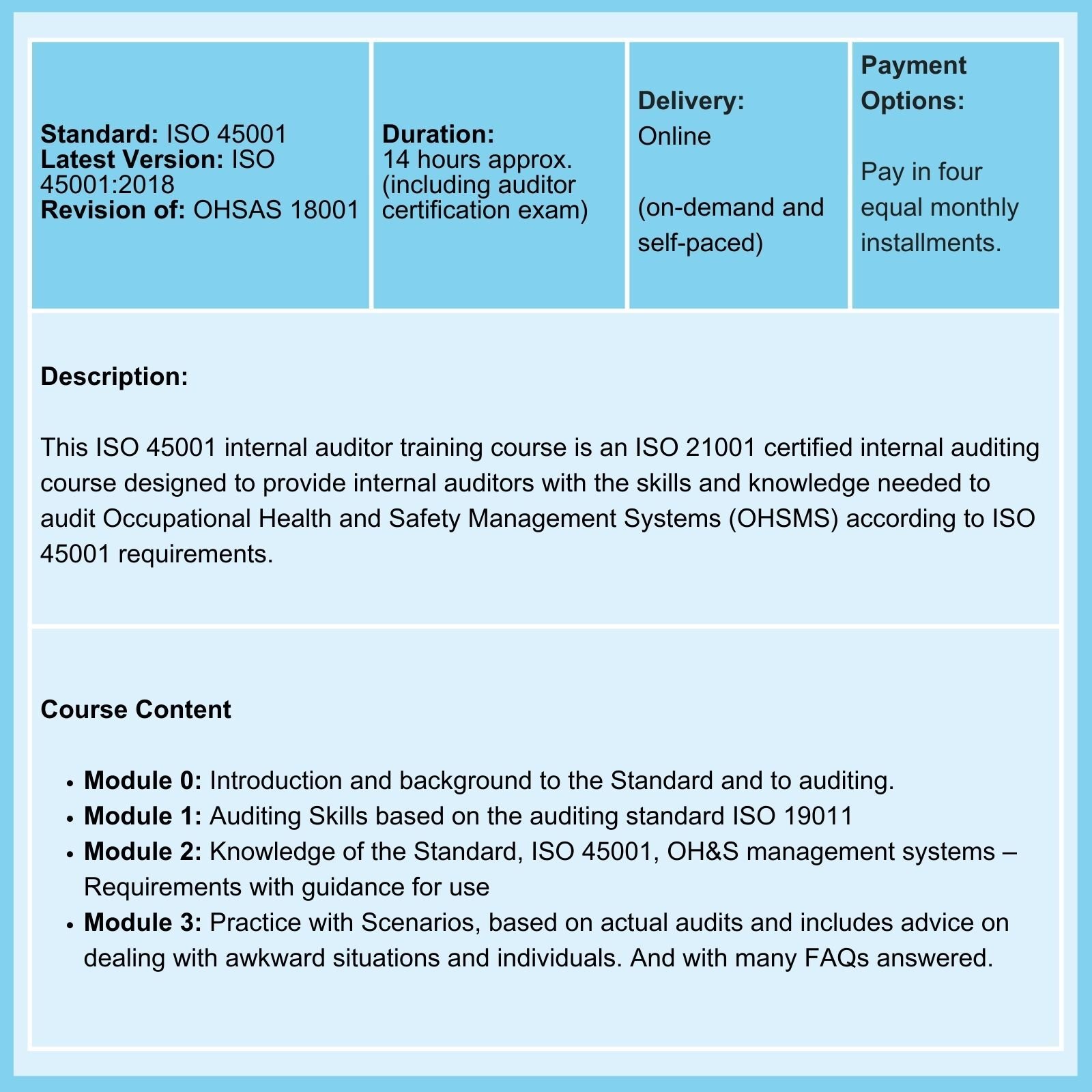
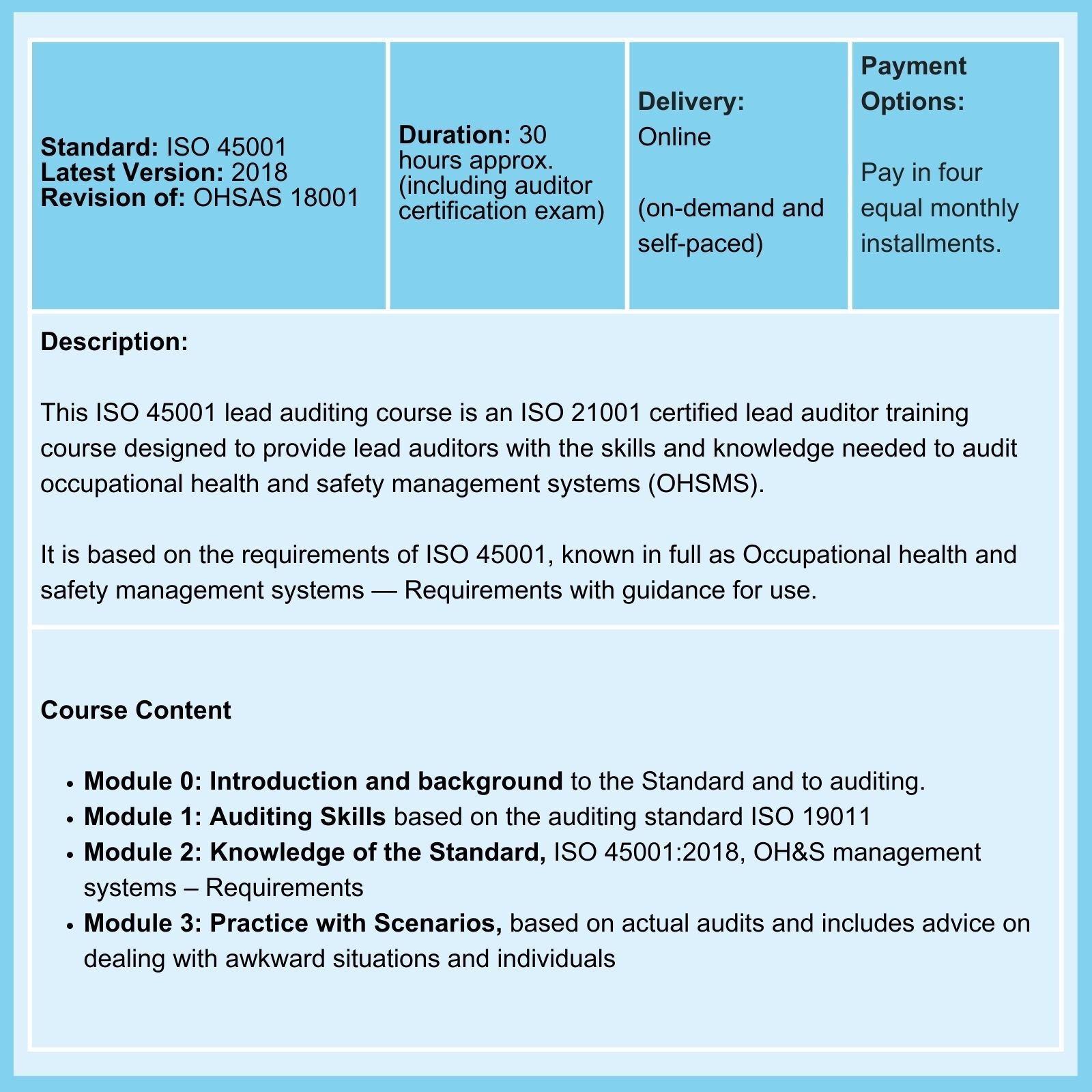
ISO 45001 |
2015 |
Mar 2027 |
While work on a revision has commenced, we await the publication of a DIS. |
|
ISO 50001 |
2018 |
None |
No immediate plan for a new version of this Energy Management System Standard. The current standard was reviewed and confirmed in 2024. |
|
ISO 55001 |
2024 |
None |
The 2024 version introduced new subclauses dealing with asset management decision-making, the strategic asset management plan, and knowledge. The 3-year transition period ends on 31-Jul-27. |
|
ISO 13485 |
2016 |
None |
While a review of the standard is currently underway, the incorporation of ISO 13485:2016 into the FDA QMSR regulations from February 2026, any revision is most unlikely before 2030. |
|
IATF 16949 |
2016 |
Sep 2027 |
The Rules 6th edition took effect Jan 2025. A revision of the Standard will follow the publication of a new edition of ISO 9001, upon which the IATF standard is based, by about 12 months. |
|
IA 9100 |
2018 |
Jan 2027 |
AS 9100 Rev D will be renamed IA 9100 when next revised. This revision will follow the publication of a new edition of ISO 900,1 upon which the standard is based. |
|
ISO 17025 |
2017 |
None |
No immediate plan for a revision of this Testing and Calibration Laboratories Standard. |
|
ISO 15189 |
2022 |
None |
No immediate plan for a revision of this Medical Laboratories Standard. The 3-year transition period ends on 31-Dec-25. |
|
ISO 19011 |
2018 |
Apr 2026 |
Currently at the FDIS stage, the revised standard will provide guidance on remote and hybrid audits and the use of digital tools to reduce travel requirements and expand audit reach. |
Looking Ahead
We will continue to monitor the publication of new Standards and progressively update this Post.
Related Courses
deGRANDSON Global is an ISO Certified Educational Organization
In October 2021, we secured certification for three education-related ISO Standards. We now have a university-grade management system in place, conforming to the requirements of …
We have chosen ISO 21001 certification because it is based on an independent third-party assessment, unlike IRCA and Exemplar badges (which we believe are commercially compromised). It is a ‘'university grade' standard in use globally by schools, colleges, and universities to demonstrate their competence.
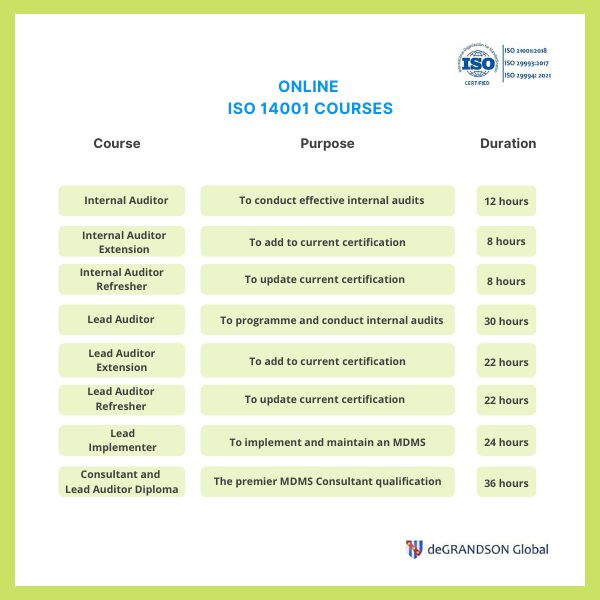
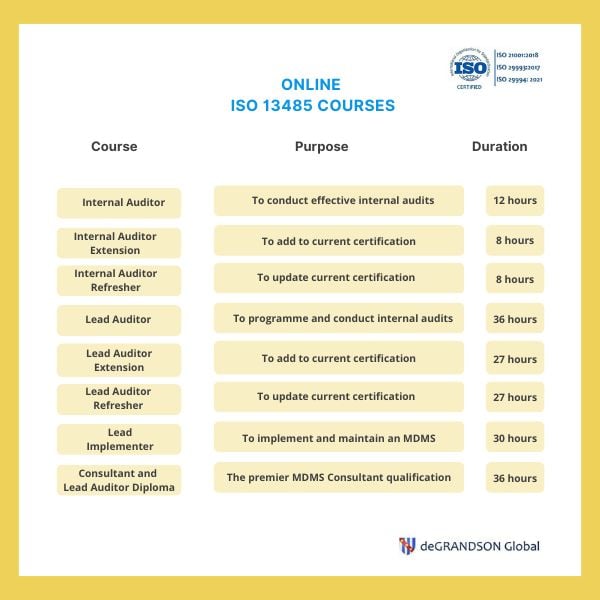
We provide Courses for ISO 9001, ISO 13485, ISO 14001, ISO 17025, ISO 27001, ISO 45001, Risk Management, Data Protection, and more.