Practical advice on compliance with Clause 9.3 requirements: The periodic management system review is an opportunity not to be missed
ISO 9001 Clause 9.3.1 requires that 'Top management shall review the organization's quality management system, at planned intervals, to ensure its continuing suitability, adequacy, and effectiveness.'
Is this just a 'rubber stamp' activity, or would you like to benefit from the requirement? If your answer is the latter, here are some examples of how to make the most of periodic ISO 9001 review meetings.
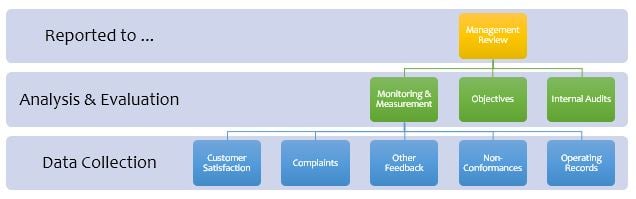
The diagram shows that Management Review is the top-level control and management activity common to all ISO management system standards. As such, it must be done well for the organization to gain the maximum benefit from the significant resources invested in implementing and maintaining ISO Certification.
Here are some examples of DOs and DONTs regarding Management Reviews and getting the most out of compliance with the requirements.
Do's and Don'ts of Performing Management Reviews
DO's
- Do take full advantage of the Process Approach. Check that your Process Map is up to date. Have any new processes or procedures been introduced this past year? What do internal and external audits and the analysis of non-conformances tell you about how well processes are controlled? Which ones have the most significant risk for the organization, where additional controls are needed, and where the critical processes are accurately identified? A brainstorming session to review and revise a process map might be very rewarding.
- Apply risk management methods progressively to all critical processes. Review your risk assessment and treatment plans against outcomes - again, the results of the internal and external audit - and the analysis of non-conformances. If you're not using formal risk assessment and risk treatment, now is the time to start. While it is not a requirement of ISO 9001, there are real business benefits, mistakes to be avoided, and profits to be made. Focus on the critical processes that are most likely to affect customer satisfaction. Risk assessments are 'live' documents and must be updated regarding experience and changes in the organization, its products and services, customer requirements, etc. But don't underestimate how much time and effort is needed to create and later revise risk assessments. Prioritize your critical processes and plan sessions once a week or once a month to address them.
- Do integrate Quality Objectives. If your organization has KPIs (Key Performance Indicators), don't consider them different from Quality Objectives. That's because KPIs are quality objectives, and their integration into your quality system will build credibility in their usefulness, especially for those who view them solely as a burden on the business.
- Do introduce adequate Calibration Control (where applicable). Inadequate calibration control is a common weakness for many businesses for whom accurate monitoring and measurement are critical to success. Too often, no dependable records justify the adequacy of the measurement devices chosen. The frequency of recalibration is dictated by calibration laboratories to maximize income and/or suit the schedules of calibration technicians (if too infrequent, erroneous results may be recorded). If either of these situations applies to your organization, you must act. In particular, you should consider the need for Switching Rules to a higher or lower frequency of calibration checks based on the history of adjustments to calibrated devices.
- Do exploit your Internal Auditor training, especially regarding improvement. Internal auditing should not be a 'box-ticking' exercise but an engagement between the auditor and the interviewees to confirm compliance with requirements (the primary purpose of the audits—see ISO 9001 Courses) and elicit suggestions for improvement. The audits are one of the few times in the year that the opinions of those most intimately involved in the organization's day-to-day work can be collected. The opportunity is there, so make sure you maximize it.
- Do promote your QMS Certification and the capabilities and Customer Satisfaction it affirms. This may seem obvious, but it is surprising how often organizations do not publicize their ISO Certification on promotional websites, publicity materials, and elsewhere. With prospective customers especially, your Certification goes beyond being a piece of paper and offers independent proof of your organization's capabilities.
Marketing and sales personnel should use ISO Certification in this context. ISO Certification is not the 'property' of the Quality Department. It would be best to ensure that every function exploits its potential for improved business. The Management Review Meeting is an ideal opportunity to consider maximizing the benefits of ISO 9001 Certification.
DON'Ts
- Don't conduct a Management Review without a written agenda covering all the mandated inputs, outputs, and other requirements of Clause 9.3.
- Don't hold the Review Meeting without top management present. They must decide whether the management system's performance is satisfactory, decide on improvement actions required, and commit any additional resources needed.
- Don't circulate reports at the Review Meeting, as it's too late. Instead, issue reports seven days before the meeting so that attendees have time to study them, identify concerns, and consider improvement options.
To conclude:
We hope you find some of these improvement suggestions helpful. Perhaps they will 'spark' other possibilities for you and provide some 'food for thought.'
Related Courses
Related Courses
Related Articles
- ISO 9001 Clauses
- ISO 9001 Consultancy: How to be a QMS Consultant?
- [Update] Seventeen official ISO 9001 Guidance Documents
- Free ISO 9001 Implementation Handbook (100+ pages)
deGRANDSON Global is an ISO Certified Educational Organization
In October 2021, we secured Certification for three education-related ISO Standards. We now have a university-grade management system in place conforming to the requirements of …
October 2021, we secured Certification for three education-related ISO Standards. We now have a university-grade management system in place conforming to the requirements of …
We have chosen ISO 21001 certification because it is based on independent third-party assessment, unlike IRCA and Exemplar badges (which we believe are commercially compromised). It is a 'university grade' standard globally by schools, colleges, and universities to demonstrate competence.
We provide ISO9001, ISO 13485, ISO 14001, ISO 17025, ISO 27001, ISO 45001, GDPR, Risk Management, and other Courses.











