But it’s complicated, required deadlines vary by Class and for all devices. You need to take action now or risk having no product to sell.
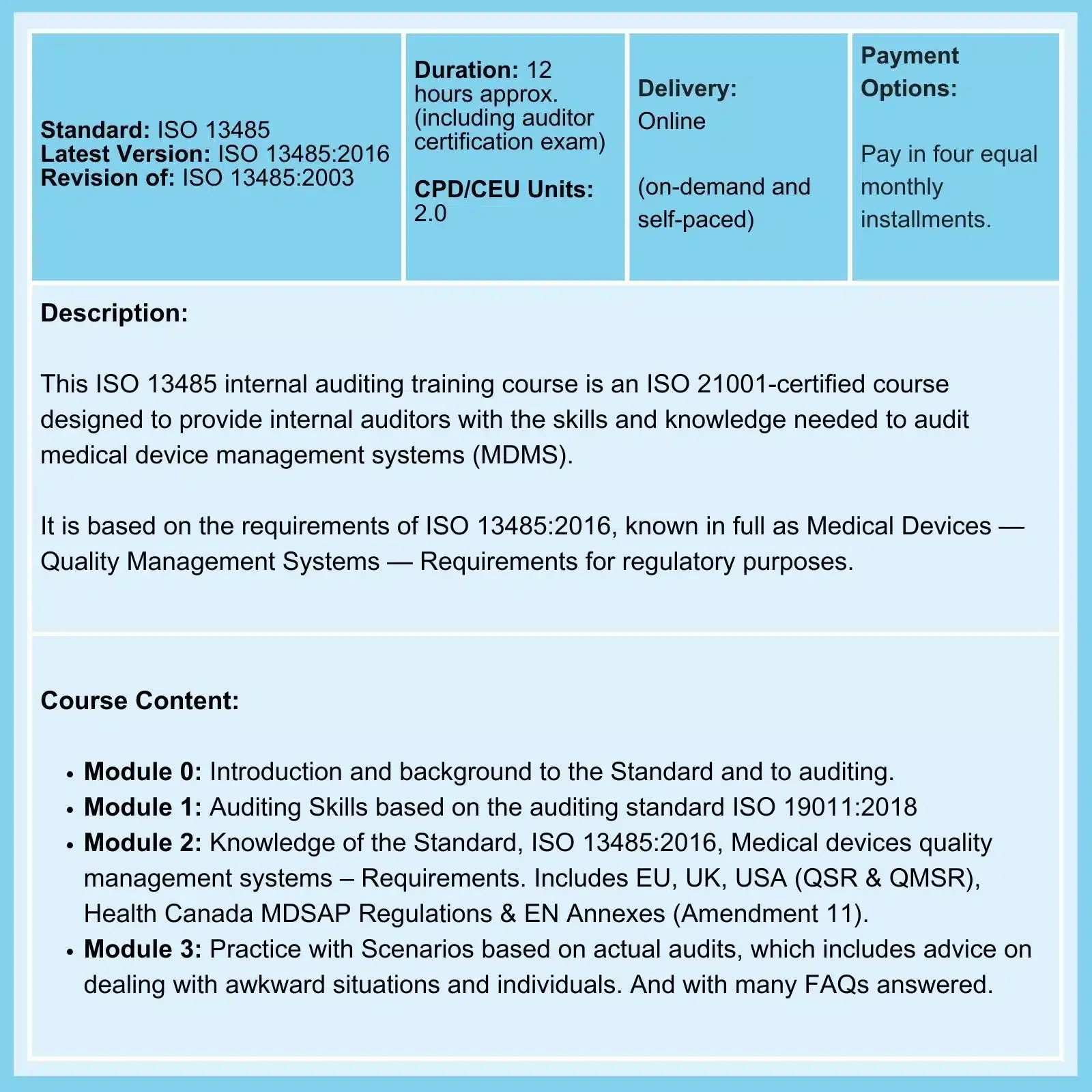
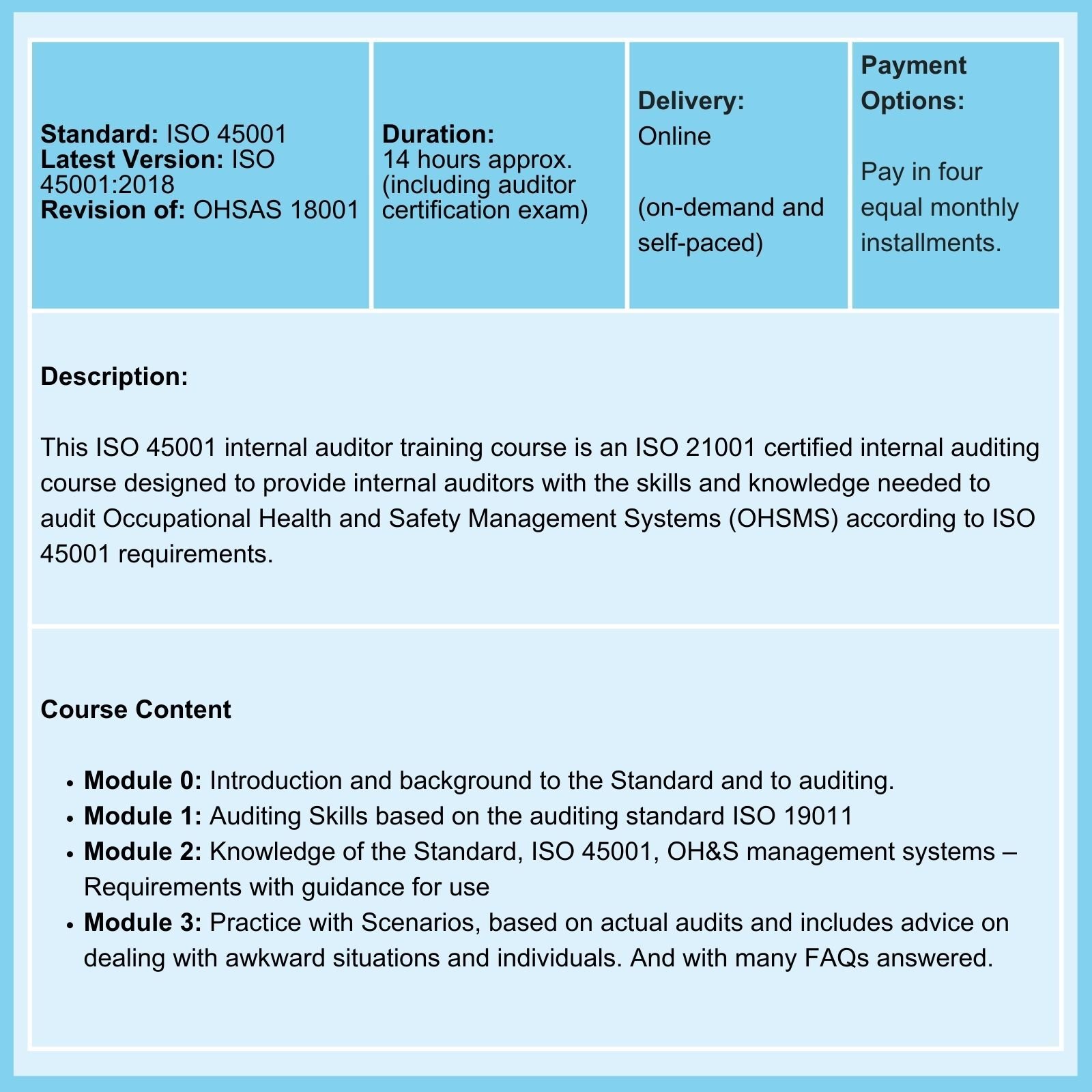
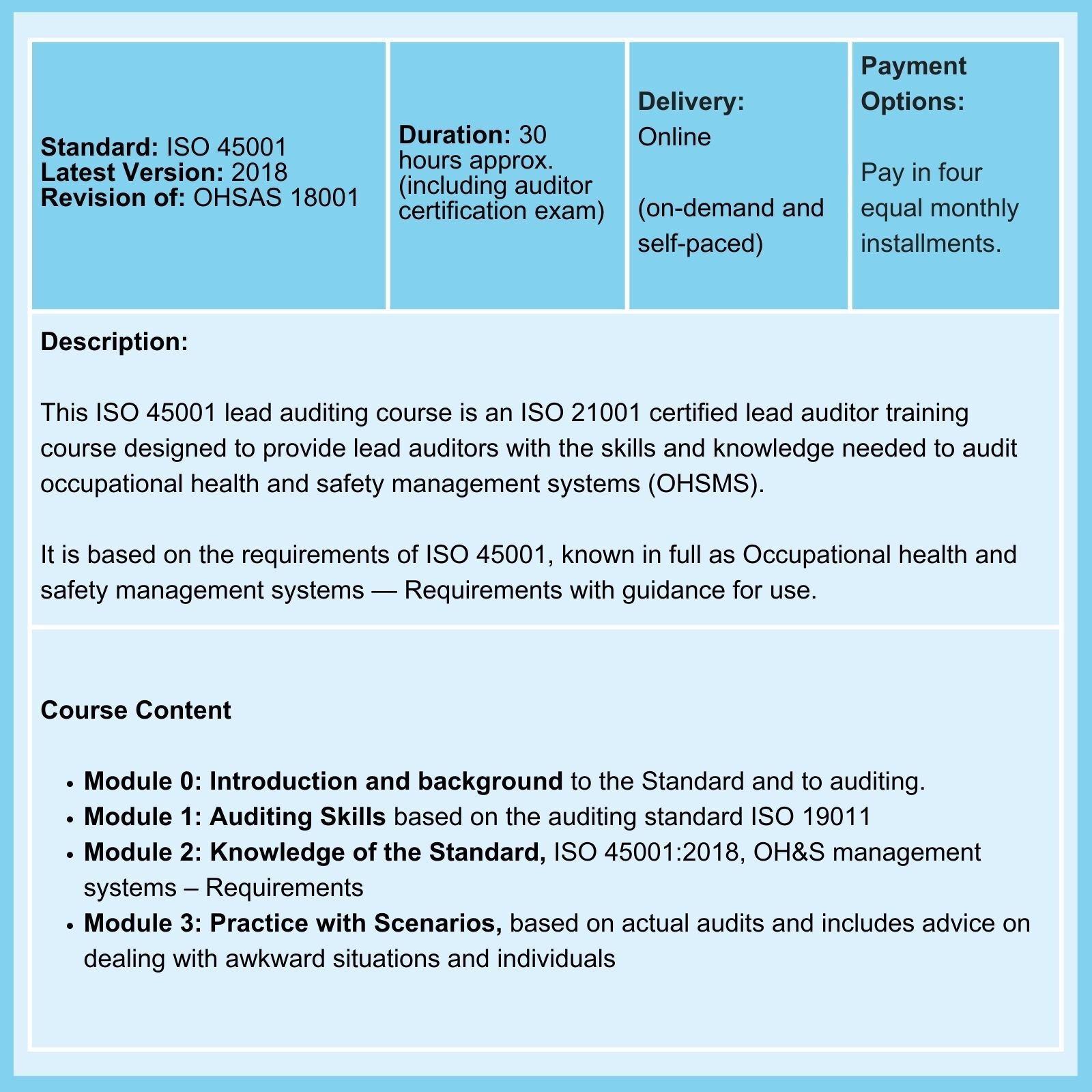
MDR Transition Overview
Under EU Medical Device Regulations (MDR) of 2017, all medical devices have to conform to the new regulations by the end of the current transition period on May 26, 2024, But because, among other things, Notified Bodies suffer from capacity problems to certify old and new medical devices, there is a risk that at the end of the current transition period, a large number of devices will no longer be available to EU patients.
A decision to extend the implementation period has therefore been taken and it officially comes into force on the day of its publication in the Official Journal of the EU, which is expected before the end of March 2023.
Note: As emphasized in our ISO 13485 training courses, compliance with applicable statutory and regulatory requirements, as applicable, is a requirement of ISO 13485 compliance.
The changes include an extension of the time periods in Article 120(2), (3) and (4) MDR and are summarised as…
- Legacy Class III and IIb implants: new transition period until 31 December 2027
- Legacy Class IIb and lower: new transition period until 31 December 2028
- Class III custom-made products: new transition period until 31 December 2026
- 'Sell-off' deadline removed
For part of the Class I products, the (old) transitional period until 26 May 2024, which is regulated in Article 120(1) MDR, remains in place (see MDCG 2020-2 rev. 1, Class I Transitional provisions under Article 120 (3 and 4) – (MDR)).
It’s complicated – get Professional Advice
With more than 10,000 types of medical device available to patients over multiple classifications, it would be impossible for a post such as this to cover all possibilities. Our purpose is to advise you of the deadlines that will now apply together with identifying (in the Explanatory Notes) the issues most likely to hinder you in complying with the EU Regulations within the time available.
You must develop (or update) a plan for compliance with the Regulations. And you are strongly advised to get professional Regulatory Affairs support in preparing and implementing that plan.
|
Device Class |
Action |
Deadline |
CE Mark by |
Comment |
|
|
|
|
|
|
|
All |
Establish a quality management system in accordance with Article 10(9) MDR |
26-May-24 |
Various |
ISO 13485 certification is the de facto requirement here |
|
Class III, IIb & IIa |
Submit formal application in accordance with Annex VII to the MDR for conformity assessment |
26-May-24 |
Notified Body |
Previous willingness by a Notified Body to accept an application must be established. |
|
Class III, IIb & IIa |
Signed agreement between Notified Body and the Manufacturer |
26-Sep-24 |
Notified Body |
|
|
Class III Customized |
MDR-compliant CE Mark Certificate |
31-Dec-26 |
Notified Body |
|
|
Class IIb |
MDR-compliant CE Mark Certificate |
31-Dec-27 |
Notified Body |
|
|
Class III |
MDR-compliant CE Mark Certificate |
31-Dec-27 |
Notified Body |
|
|
Class I |
MDR-compliant CE Mark Certificate |
31-Dec-28 |
Self-declared |
Follow MDCG 2020-2 rev. 1 - Class I Transitional provisions under Article 120 (3 and 4) - (MDR) |
|
Class IIa |
MDR-compliant CE Mark Certificate |
31-Dec-28 |
Notified Body |
|
Explanatory Notes
MDR-compliant CE Mark Certificate extensions: These deadline extensions will be dependent on the fulfillment of several conditions and the extension of the validity of current MDD and AIMDD CE certificates that would only apply to devices…
- That do not present any unacceptable risk to health and safety. That have not undergone significant changes in design or intended purpose.
- For which the manufacturers have already undertaken the necessary steps to transition to certification under the EU MDR.
Continuing to meet the requirements of EU-MDR for legacy devices: To maintain compliance with EU-MDR during the extended transition period, you must…
- Continue to comply with the MDD or AIMDD requirements. Devices covered by a certificate issued under either MDD or AIMDD will be kept under surveillance, either by the notified body that issued the certificate(s) or a notified body designated under the EU MDR. This, and the market surveillance activities of the competent authority, will monitor whether the device presents an “unacceptable risk to health and safety.”
- Ensure that there has been no significant change in design or intended purpose of the device(s) since May 2021, nor can any significant changes be made until certification under EU MDR has been completed.
- Adapt the quality management system (QMS) to meet the requirements of Article 10(9) by no later than May 26, 2024. In submitting an application for conformity assessment to a notified body, the manufacturer is declaring that the QMS is compliant with the EU MDR.
- Ensure that an application has been lodged by either the manufacturer or authorized representative to a designated notified body prior to the expiration of the CE certificate issued under the MDD and AIMDD, or by no later than May 26, 2024. The manufacturer and the notified body must have signed a written agreement by no later than September 26, 2024. Only devices that the manufacturer intends to transition to the EU MDR may benefit from the extended transition period. If the manufacturer intends to replace legacy devices with a “new” device under the EU MDR, the manufacturer must apply for conformity assessment for the new device prior to May 26, 2024, for the extension to be applicable to the legacy device.
- Confirm continuous application of EU MDR requirements in relation to postmarket surveillance (PMS), vigilance, and market surveillance.
- Take note of the Sell-off Date Rule change: The “sell-off” date is the end date after which devices already on the market but not yet with the final user should be withdrawn. The removal of the ‘sell-off’ deadline ensures that the medical devices concerned continue to be available beyond the end date of 26 May 2025.
- Follow MDCG- endorsed documents and other guidance: The Medical Device Coordination Group (MDCG) deals with key issues from the medical devices sector, from Notified Body oversight or standardization to market surveillance, passing by international matters, new technologies, and clinical investigation. The MDCG provides an official, though not legally-binding, interpretation of MDR and IVDR. The regular series of papers produced offer valuable advice, especially for borderline situations. Learn more information on MDCG here.
- Watch the development of the EUDAMED Database carefully: Once finalized the entry of manufacturers’ product information onto the EUDAMED Database will become mandatory. Development of version 1 of EUDAMED will end in Q4 2023. This will be followed by a regulation-mandated audit of EUDAMED happening in Q1 2024 with the MDCG sign-off in Q2 2024.
EUDAMED will have six Modules, namely,
- Actor and User Registration and Management.
- UDI Database and Registration of Devices.
- Certificates and Notified Bodies.
- Clinical Investigation and Performance Studies.
- Vigilance and Post-market Surveillance.
- Market Surveillance.
The first three of these are already available for use and you are recommended to start uploading data as soon as possible.
No recent announcements regarding further changes to IVDR Transition deadlines
The deadlines here remain as given in our Post: IVDR Transition Deadline extended up to 2027 – reality bites.
Conclusion: You need to act/react now
The extension given is to facilitate the work of Notified Bodies and provide a reasonable opportunity for them to clear the backlog of CE Mark applications. It is not for the benefit of manufacturers or their EU representatives. If you doubt this, look again at the table of deadlines. If you’ve not already done so, you need to take action now. Some Notified Bodies are reported to be refusing new applications. So, don’t get caught out!
Related Courses
Related Courses
Related Articles
- Medical Device Regulations (MDR) Classifications: US vs EU
- ISO 13485 Certification: 26 FAQs answered (downloadable PDF file attached)
deGRANDSON Global is an ISO Certified Educational Organization
We have chosen ISO 21001 certification because, unlike IRCA and Exemplar badges (which in our opinion are commercially compromised), it is based on independent third-party assessment. It is a ‘university grade’ standard in use globally by schools, colleges, and universities to demonstrate their competence.