The proposed EU MDR changes for Software as a Medical Device (SaMD) will be revolutionary.
And will be a relief for SMEs, University spin-offs, and Start-Ups, for whom a 6-figure buy-in for Notified Body services has been an insurmountable barrier. Currently, Europe-based developers of SaMD and those in other locations are avoiding the EU market and initially launching their products in the USA and the UK, where market-entry costs are much lower. The result is that European citizens who would benefit from these products are deprived of access to them.
This news has lain unnoticed since it entered the public domain in December 2025. Most attention has focused on an overall reduction in the burden of engaging a Notified Body and obtaining CE Marks for medical devices sold in the European market.
Here's the good news…
The European Commission published a formal proposal on December 16, 2025, to revise the Medical Device Regulation (MDR), specifically targeting the "notorious" Rule 11. This proposed amendment aims to streamline software classification by adopting a more proportionate, risk-based approach aligned with international standards.
As a result, the vast majority of SaMD products will be classified as Class 1; Notified Body services will not be required, and manufacturers will issue CE Mark certificates of conformity via self-declaration.
Key Changes to Rule 11
The proposed revision reverses the current logic under which almost all software is up-classified to Class IIa or higher.
- Class I as the Starting Point: Under the new draft, software intended to confer a clinical benefit is classified as Class I by default.
- Risk-Based Escalation: Software is only "up-classified" if it meets specific criteria based on the clinical situation's severity and the significance of the information provided:
- Class III: For "critical" situations where an output error could cause death or irreversible health deterioration.
- Class IIb: For "serious" situations involving risk of serious deterioration or surgical intervention or driving management in critical situations.
- Class IIa: For "non-serious" situations or informing management in serious/critical scenarios.
- International Alignment: The wording is intended to align closely with the International Medical Device Regulators Forum (IMDRF) framework for Software as a Medical Device (SaMD).
Wider Regulatory Impact
- Lowered Barriers for Innovation: By potentially allowing more digital health applications (DiGAs) and low-risk tools to remain in Class I, the revision aims to reduce the administrative burden and costs of involving Notified Bodies.
- EU AI Act Interaction: The proposal may clarify and potentially reduce the number of AI-enabled medical devices deemed "high risk" under the EU AI Act, avoiding duplicative conformity assessments.
- Cybersecurity & Transparency: The draft integrates explicit cybersecurity requirements into the General Safety and Performance Requirements (GSPR), requiring manufacturers to report vulnerabilities to ENISA.
Current Status and Timeline
- Legislative Process: The proposal is currently in the early stages of the European legislative process and must be reviewed and adopted by the European Parliament and Council.
- Effective Date: Experts do not expect these changes to take effect until 2027 at the earliest, as the amendment process typically spans several years.
Meanwhile, until formally adopted, the current, stricter Rule 11 remains in full effect. Manufacturers are advised against prematurely changing existing compliance plans.
Note: A detailed and informed review of all proposed changes to EU MRD/IVDR can be found on the Lexology website.
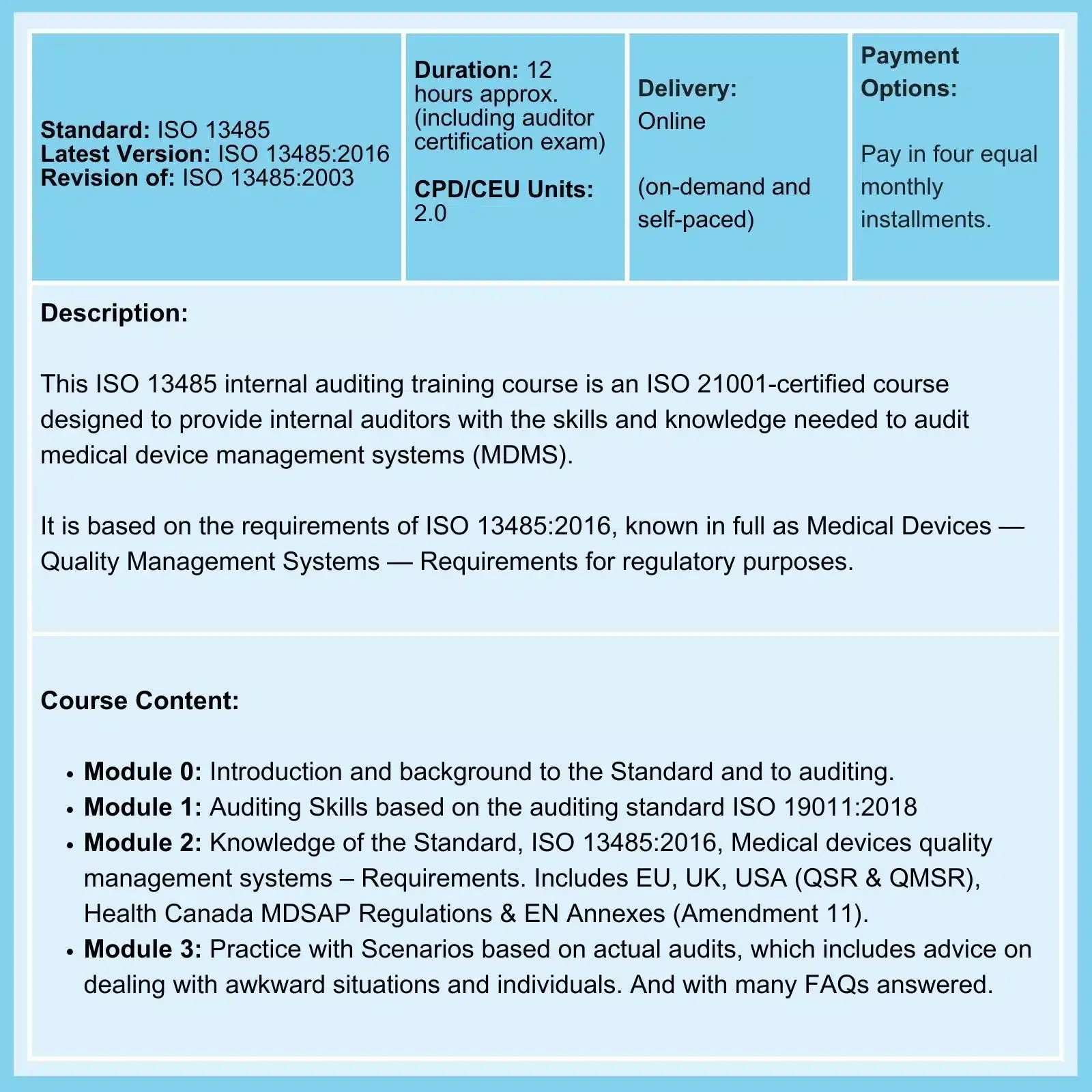
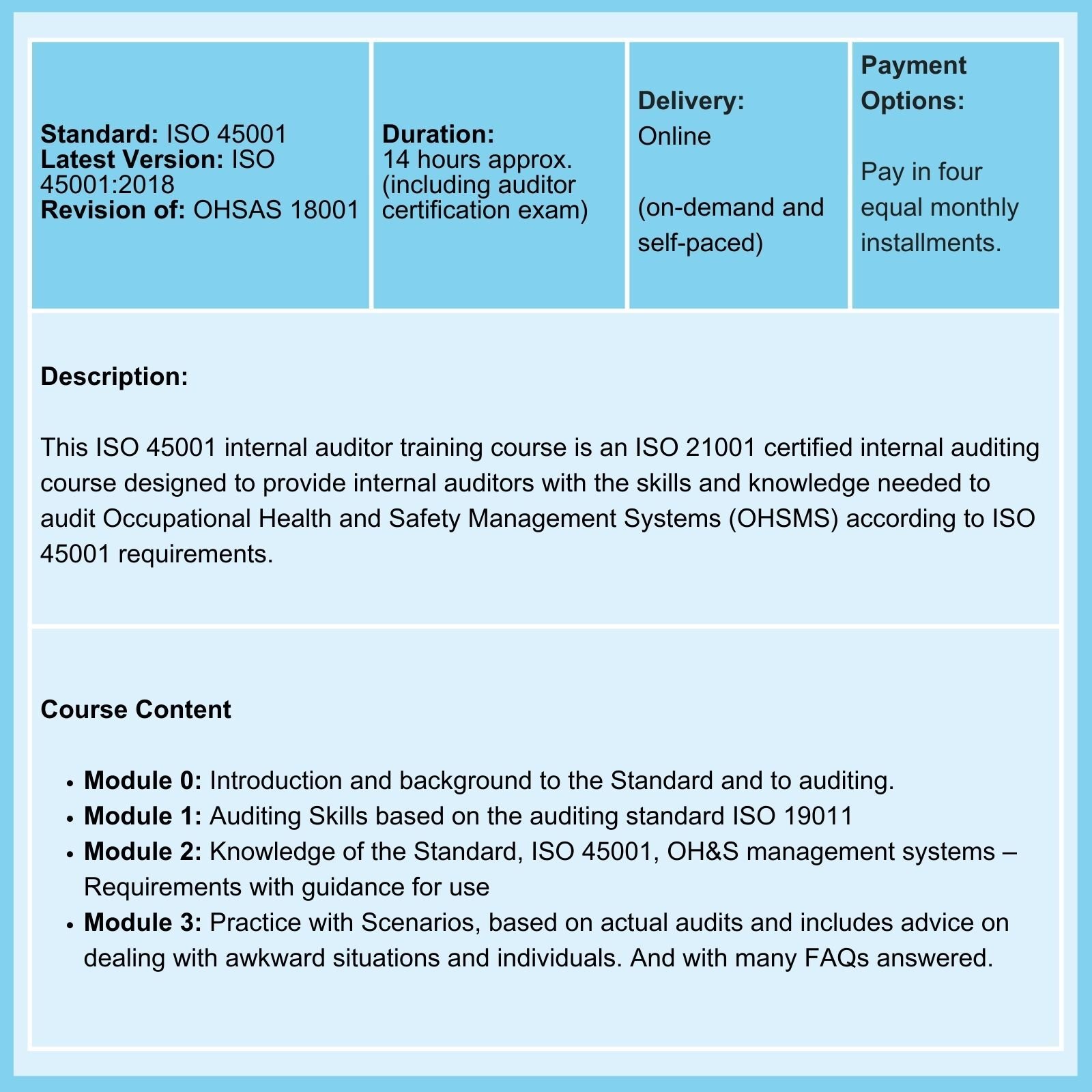
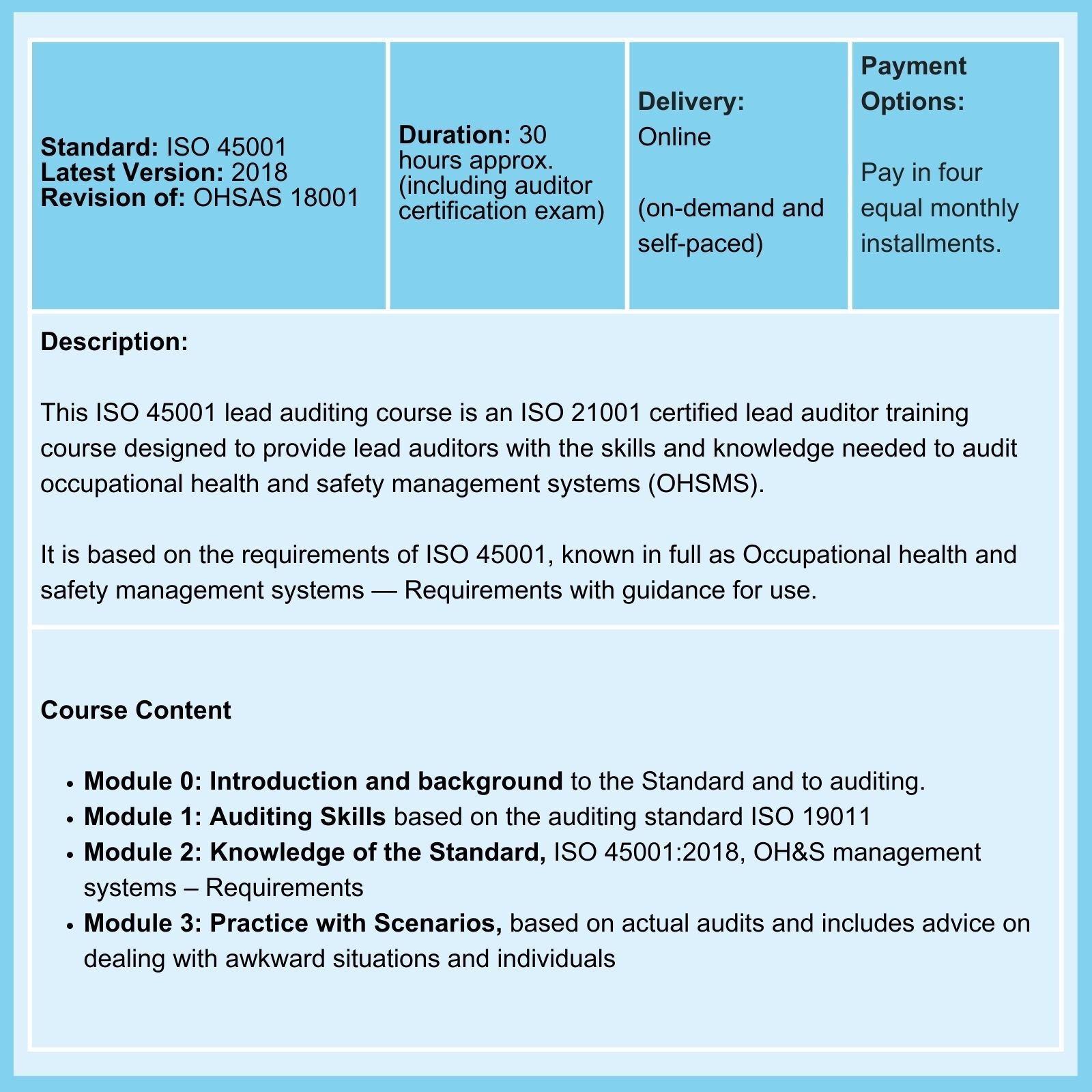
Related Courses
deGRANDSON Global is an ISO-Certified Educational Organization
In October 2021, we secured certification for three education-related ISO Standards. As a result, we now have a university-grade management system that conforms to the requirements of …
October 2021, we secured certification for three education-related ISO Standards. As a result, we now have a university-grade management system that conforms to the requirements of …
We have chosen ISO 21001 certification because, unlike IRCA and Exemplar badges (which we believe are commercially compromised), it is based on an independent third-party assessment. In addition, it is a 'university grade' standard used globally by schools, colleges, and universities to demonstrate competence.
We offer Certified Courses for ISO 9001, ISO 13485, ISO 14001, ISO 17025, ISO 27001, ISO 45001, Data Protection, and Risk Management.