The EU MDCG 2019-7 provides guidance on "Persons Responsible for Regulatory Compliance’ (PRRC).
The European Commission (EC) has published MDCG 2019-7, Guidance on Article 15 of the Medical Device Regulation (MDR) and in vitro Diagnostic Device Regulation (IVDR) regarding a ‘person responsible for regulatory compliance’ (PRRC).
Meeting the requirements here will challenge SMEs and start-ups, but the guidance offers practical assistance. Most SMEs and start-ups appear unaware they are breaching the Regulations, especially as ISO 13485 training may not have addressed EU Regulatory requirements.
Guidelines Regarding the Role of the PRRC
The role of PRRC is mandated in Article 15 of both the EU MDR and EU IVDR. These regulations require all manufacturers and Authorized Representatives to have a designated employee in their company who is responsible for regulatory compliance with the applicable MDR or IVDR requirements.
NOTE: Medical Device Component Manufacturers, logistics companies, and all others who are not Medical Device Manufacturers or Authorised Representatives as defined in MDR/IVDR are NOT required to have PRRC.
MDR and IVDR outline five major responsibilities for a PRRC:
-
The PRRC will check the conformity of devices in accordance with the quality management system (QMS) the company uses before the device is released.
-
PRRC will ensure that technical documentation and the EU documentation of conformity are generated and up-to-date.
-
The PRRC will ensure the company complies with post-market surveillance obligations listed in Article 10(10) of MDR and Article 10(9) of IVDR.
-
Additionally, the PRRC will ensure that the company fulfills its reporting obligations, found in Articles 87-91 of MDR and Articles 82-86 of IVDR.
-
Finally, the PRRC will ensure that the statement from Section 4.1 of Chapter II of Annex XV of the MDR is issued if the company is building an investigational device. For the IVDR, this statement is located in Section 4.1 of Annex XIV.
Minimum Qualifications for a PRRC.
The prerequisite expertise for a PRRC, as set out in the new regulations, must fulfill either of the following criteria:
- a diploma, certificate, or other evidence of formal qualification awarded on completion of a university degree or of a course of study recognized as equivalent by the Member State concerned in law, medicine, pharmacy, engineering, or another relevant scientific discipline, and at least one year of professional experience in regulatory affairs or in quality management systems relating to medical devices;
- four years of professional experience in regulatory affairs or in quality management systems relating to medical devices.
The MDCG Guidance offers a flexible interpretation, especially for SMEs. However, every EU manufacturer and distributor must read it carefully to determine if the PRRC requirements will be fulfilled.
Note:
1. The Medical Device Coordination Group (MDCG) Series of Guidelines is a handy and readily understandable set of continuously expanding publications. If EU Regulatory Affairs for medical devices is of interest, their website is well worth monitoring.
2. All EU Manufacturers and Authorized Representatives must comply with PRRC requirements – don’t ignore the regulations or the guidance.
3. A manufacturer can outsource the PRRC Role provided the company has fewer than 50 full-time employees and less than €10 million in global sales. Additionally, the PRRC must be “permanently and continuously” at your disposal of the company.
3. In the UK (Great Britain), there is currently no requirement for a person equivalent to the PRRC under EU Regulations.
Registration with EUDAMED
The name, address, and contact details of the person or persons responsible for regulatory compliance shall
be registered in EUDAMED (MDR Article 31 and Annex VI, Part A, clause 1.4; IVDR Article 28 and Annex VI,
Part A, clause 1.4), whose actor registration module includes an optional field to list the responsibilities
of a specific PRRC.
MDCG Guidance 2019-5, Registration of legacy devices in EUDAMED, states that legacy devices must be
registered in EUDAMED with different timelines depending on the classification and validity of existing certificates.
Therefore, an organization may need to obtain a Single Registration Number, which requires
actor registration in EUDAMED and the PRRC’s contact details before or by the date of application.
Related Courses
Explore Related ISO Courses by Type
deGRANDSON Global is an ISO Certified Educational Organization
We have chosen ISO 21001 certification because, unlike IRCA and Exemplar badges (which, in our opinion, are commercially compromised), it is based on independent third-party assessment. It is a ‘university grade’ standard globally by schools, colleges, and universities to demonstrate their competence.
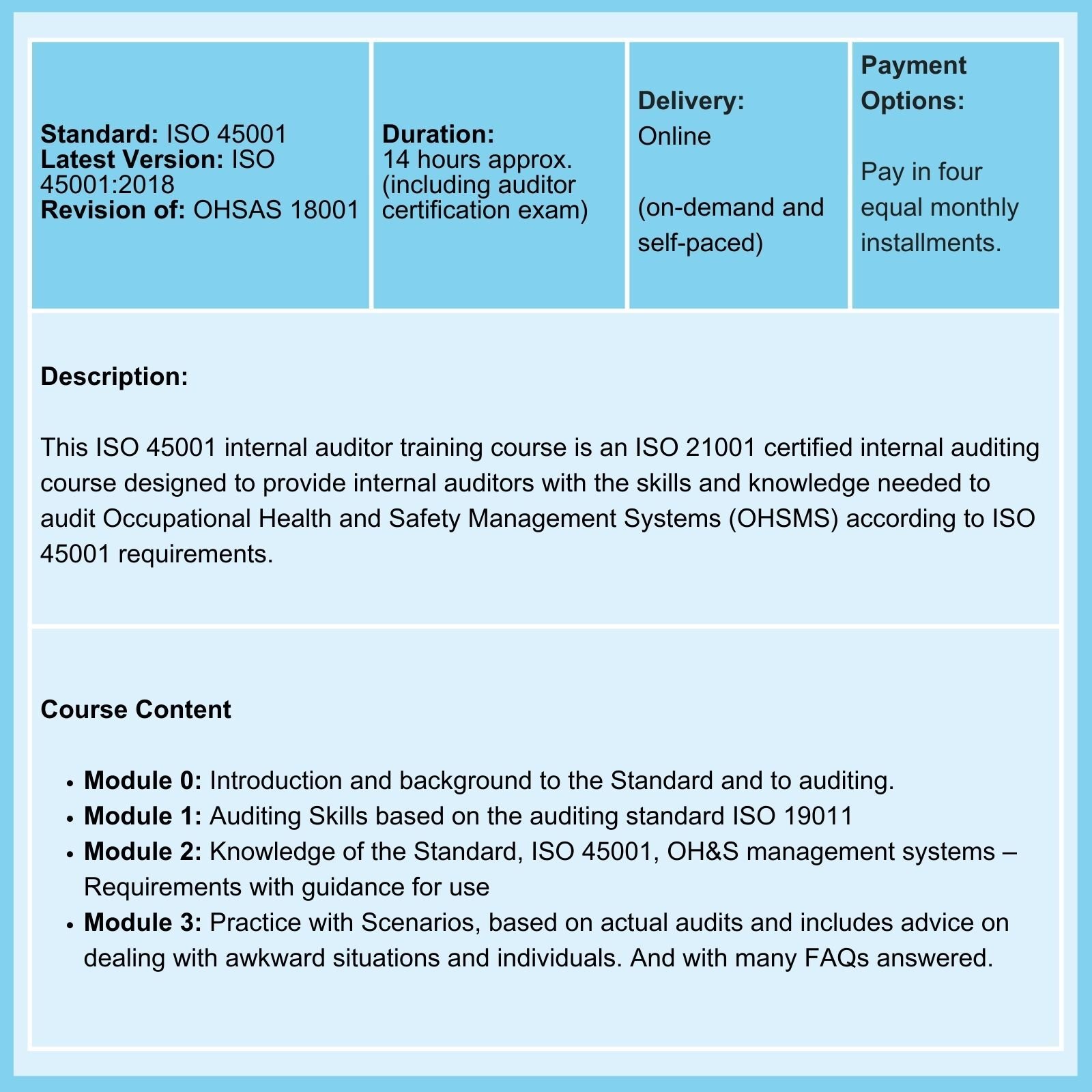
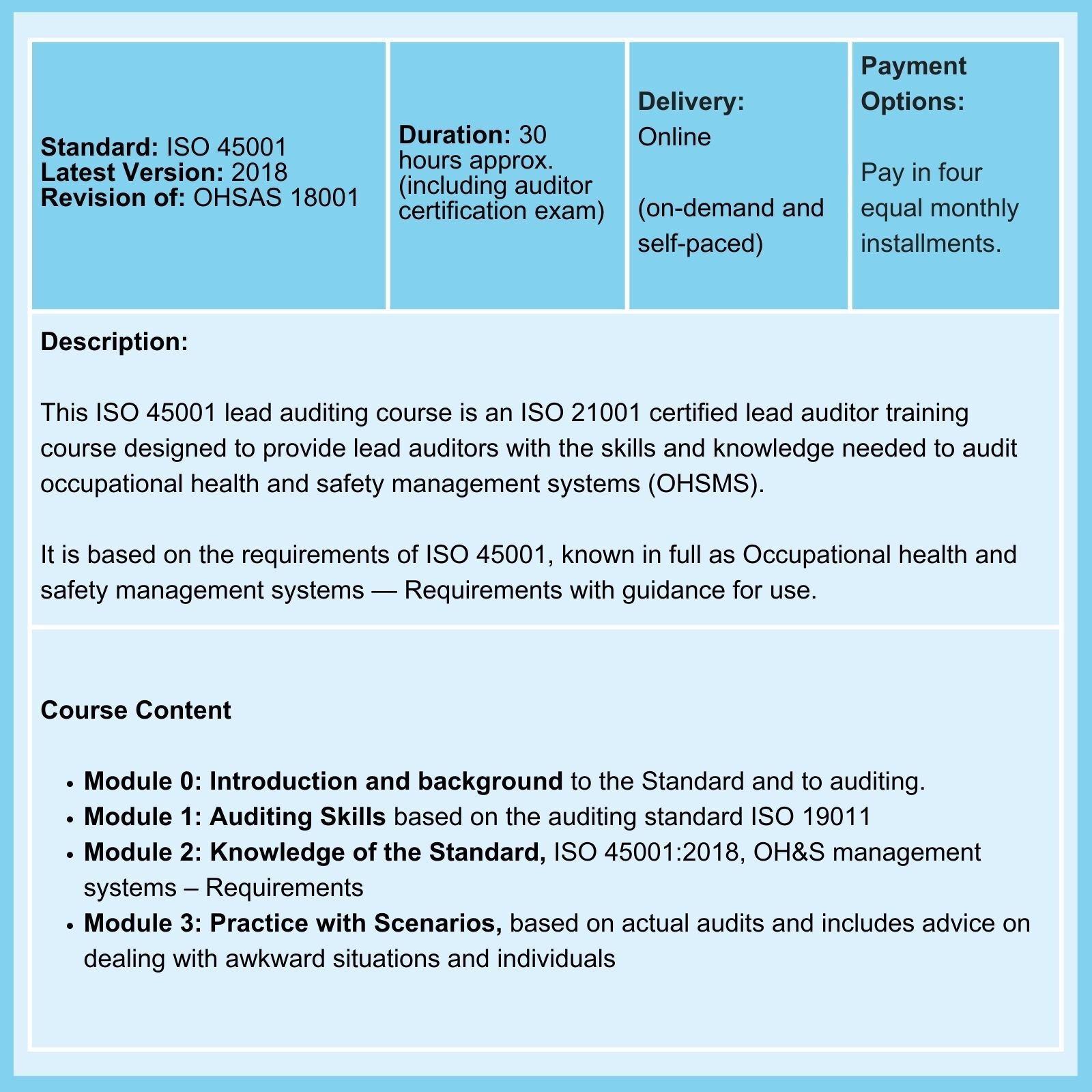
We offer Certified Courses for ISO 9001, ISO 13485, ISO 14001, ISO 17025, ISO 27001, ISO 45001, Data Protection, and Risk Management.
Written by Dr John FitzGerald
Related Articles…
The proposed EU MDR changes for Software as a Medical Device (SaMD) will be revolutionary. And will be a relief for SMEs, University spin-offs, and Start-Ups, for whom a 6-figure buy-in for Notified Body services has been an insurmountable barrier. Currently, Europe-based developers of SaMD and those in other locations are avoiding the ... Continue reading
QMSR & ISO 13485: On 02-Feb-26, the FDA Final Rule comes into force. The amendments incorporate by reference (and so align more closely with) the international standard ISO 13485:2016, Medical Devices - Quality Management Systems - Requirements for regulatory purposes. Two distinct and different requirements in ISO 13485:2016 for the ... Continue reading