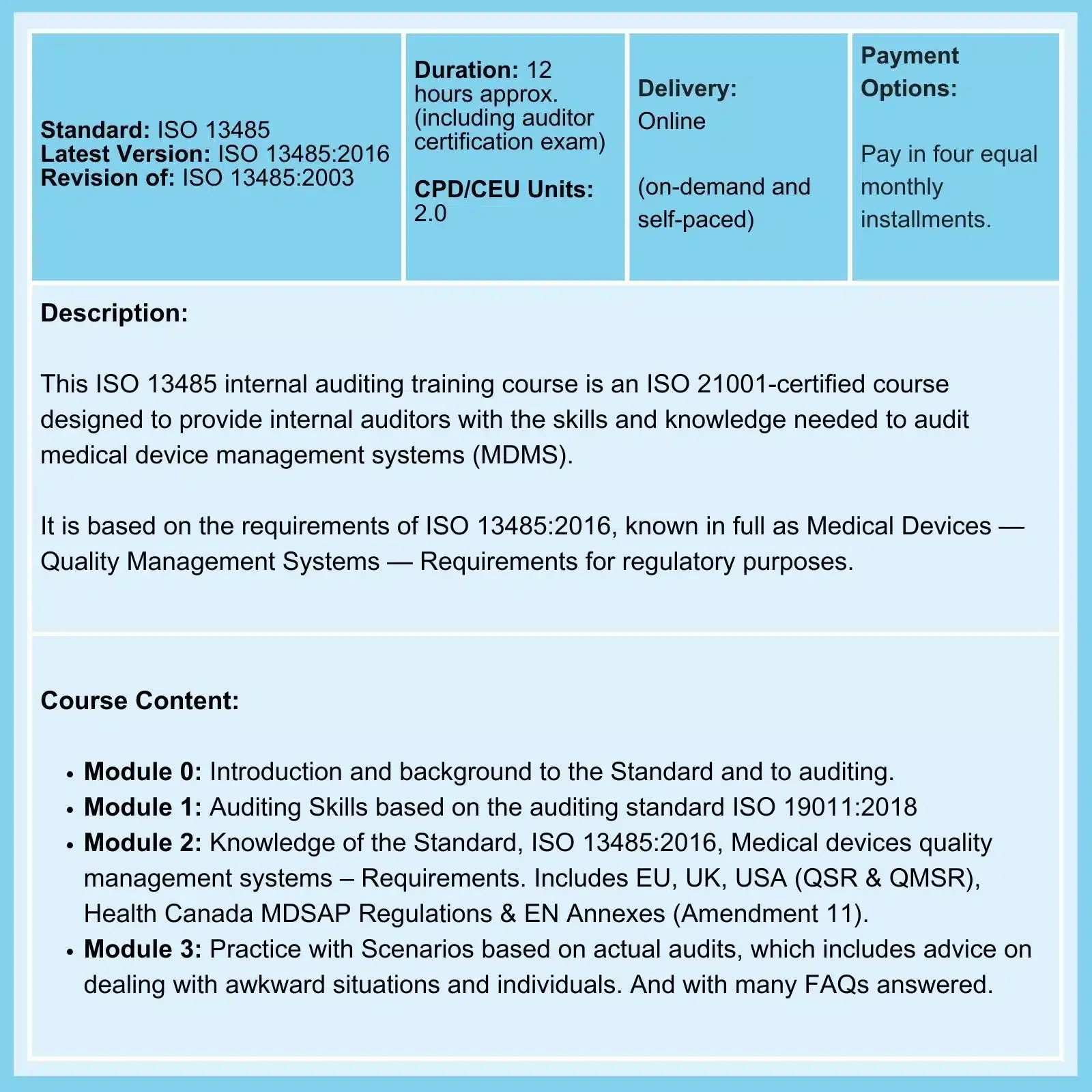
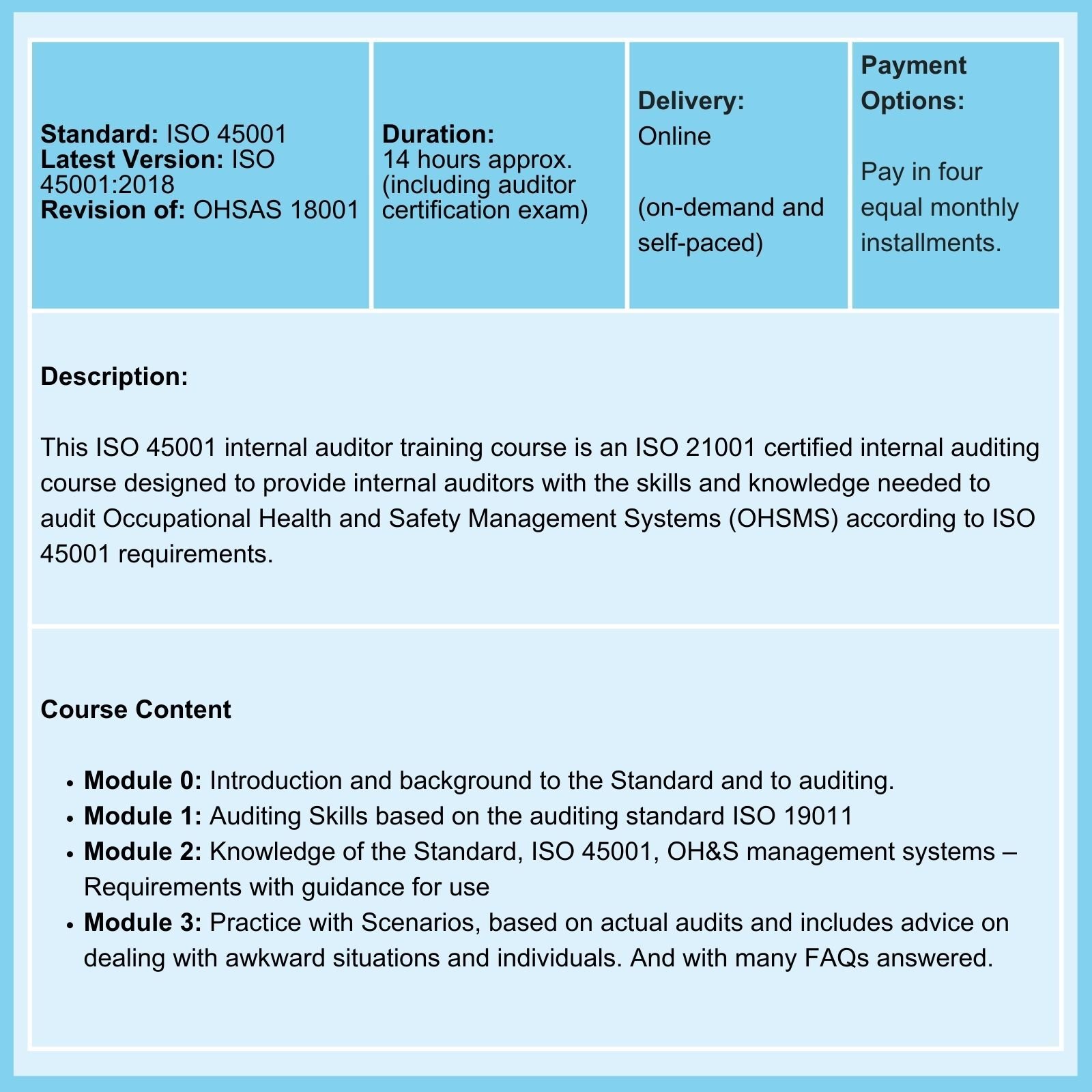
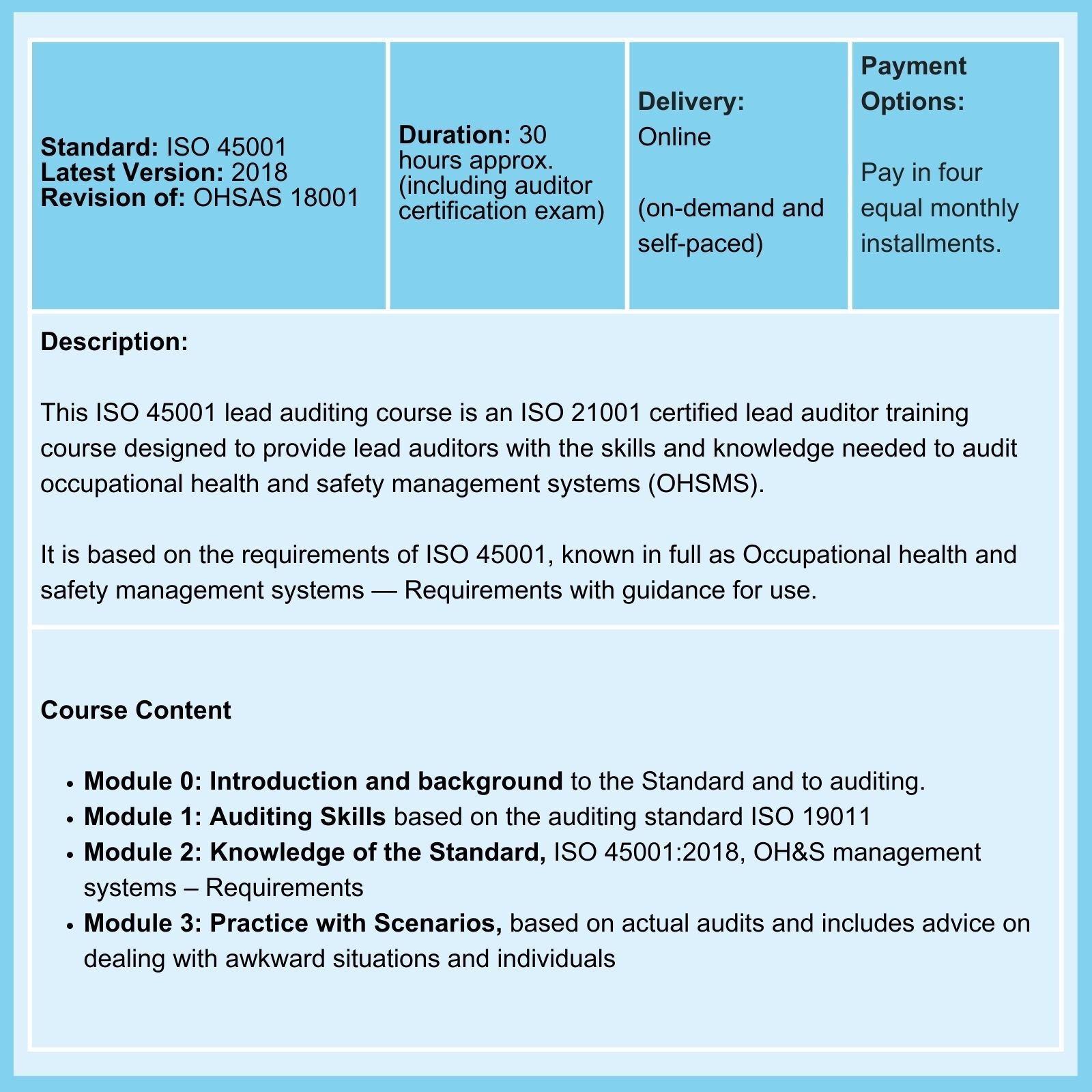
QMSR & ISO 13485: On 02-Feb-26, the FDA Final Rule comes into force. The amendments incorporate by reference (and so align more closely with) the international standard ISO 13485:2016, Medical Devices - Quality Management Systems - Requirements for regulatory purposes.
ISO 13485 Certification has the potential to greatly benefit financially all businesses involved in the Medical Device Supply Chain
While ISO 13485 certification is currently not a requirement in some countries, it is rapidly becoming a de facto worldwide regulatory requirement. You will not do business internationally or avoid unnecessary regulatory inspection without it.The ISO 13485:2016 standard is a globally recognized quality management system (QMS) designed for medical device manufacturers. It provides a framework for companies to demonstrate their commitment to meeting customer and regulatory requirements and ensuring the safety and efficacy of their products. It is also widely used within the Medical Device supply chain (e.g., component manufacturers, cold chain delivery) as many manufacturers like their suppliers to hold this certification.
And perhaps ISO 13485 training needs to be part of your preparation here.
Medical Device Regulations Around the World
In Europe
Regulatory bodies increasingly recognize ISO 13485 certification to demonstrate compliance with international quality and patient/user safety standards.
For example, in the European Union (EU), the Medical Device Regulation (MDR) requires all medical device manufacturers to comply with ISO 13485 certification to demonstrate their compliance with essential safety and performance requirements. The MDR came fully into effect on May 26, 2021, at the end of the transition period, and has significantly increased the scrutiny and requirements for medical device manufacturers. As a result, companies that comply with the MDR and associated standards and associated guidelines maintain access to the EU market, the world's second-largest market for medical devices.
In the USA
Similarly, the United States Food and Drug Administration (FDA) has recognised the ISO 13485 standard to demonstrate compliance with its Quality System Regulation (QSR) for medical devices. The FDA has stated that it expects medical device manufacturers to be certified to ISO 13485 by an accredited third-party certification body.
The new QMSR, which are mandatory from February 2, 2026, calls for incorporation by 'reference ' of the clauses of ISO 13485. In fact, the FDA has confirmed that ISO 13485 in its entirety is a requirement of QMSR. Being certified to this standard should reduce the frequency of visits by FDA Inspectors.
Other Countries
Countries such as Australia, Canada, and Japan have also recognised ISO 13485 certification to demonstrate compliance with their respective regulatory requirements. Indeed, compliance with the standard is mandatory in some countries, such as Canada and Australia, and is often a requirement for companies seeking to market their products in other countries. Furthermore, as medical device markets expand globally, more countries will likely adopt ISO 13485 certification requirements.
Continued alignment of UK Regulations with those of the EU is expected to continue (as anything else would be economic suicide). As with the EU, ISO 13485 will remain the relevant QMS standard for medical device manufacture.
MDSAP incorporates ISO 13485 Requirements
And then there is the MDSAP Scheme. The Medical Device Single Audit Program (MDSAP) allows medical device manufacturers to undergo a single audit that satisfies the regulatory requirements of multiple countries. The program was developed by the International Medical Device Regulators Forum (IMDRF), a group of regulatory bodies worldwide.
Under the MDSAP, manufacturers can undergo an audit by an authorized auditing organization that assesses their compliance with the regulatory requirements of participating countries, including Canada, Australia, Japan, Brazil, and the United States. The audit covers aspects such as QMS, regulatory, and product-specific requirements.
The MDSAP aims to streamline the regulatory process for medical device manufacturers, reduce the regulatory burden on manufacturers, and improve the consistency of regulatory requirements across participating countries. Compliance with the program is voluntary, but it is becoming increasingly popular as more countries participate. And certification to ISO 13485 is a prerequisite for MDSAP.
Note: MDSAP Certification can be up to 50 times more expensive than accredited certification to ISO 13485.
Not just a Regulatory Requirement
ISO 13485 certification is not only becoming a regulatory requirement, but it also offers significant benefits for medical device manufacturers. Certification can help companies improve their QMS, reduce costs, and enhance customer satisfaction. The standard provides a framework for companies to establish and maintain an effective QMS, including processes for design and development, risk management, and quality control. In addition, certification demonstrates to customers and stakeholders that a company is committed to meeting international quality and safety standards.
Is ISO 13485:2016 not due for a Revision?
Under the usual ISO Rules for ISO management system standards, this 2016 standard should be reviewed and revised within ten years. A revision was initiated back in 2019 but later quietly dropped. The problem is that over the past seven years or so, there have been many significant changes and proposed changes to medical device regulations, especially in Europe and the USA (not to mention Brexit). A new revision only makes sense after major regulatory changes have been completed and ‘bedded in’. So, a Revision before 2030 seems most unlikely.
The Road Ahead for ISO 13485:2016
So, ISO 13485:2016 certification is rapidly becoming a worldwide regulatory requirement for medical device manufacturers. Certification helps companies comply with regulatory requirements and provides significant benefits, including improved QMS, reduced costs, and enhanced customer satisfaction. In addition, as the global medical device market expands, more countries will likely adopt ISO 13485 certification requirements. Therefore, medical device manufacturers should consider obtaining ISO 13485 certification to remain competitive in the global market and demonstrate their commitment to quality and safety.
Related Courses
Related Articles
- FDA QMSR and ISO 13485 – what you need to know
- FDA QMSR and ISO 14971: What you need to know
- EU MDR and IVDR Guidance on PRRC Requirements
- ISO 13485: Critical Subcontractors and Crucial Suppliers
- IVDR Transition Deadline extended up to 2027 – reality bites
deGRANDSON Global is an ISO-Certified Educational Organization
In October 2021, we secured certification to three education-related ISO Standards. As a result, we now have a university-grade management system conforming to the requirements of …
October 2021, we secured certification to three education-related ISO Standards. As a result, we now have a university-grade management system conforming to the requirements of …
We have chosen ISO 21001 certification because, unlike IRCA and Exemplar badges (which we believe are commercially compromised), it is based on an independent third-party assessment. In addition, it is a ‘university grade’ standard in use globally by schools, colleges, and universities to demonstrate their competence.
We offer Certified Courses for ISO 9001, ISO 13485, ISO 14001, ISO 17025, ISO 27001, ISO 45001, Data Protection, and Risk Management.